What is SpaceOAR™ Hydrogel?
SpaceOAR Hydrogel is an absorbable gel material that creates a temporary space between the prostate and the rectum, potentially reducing radiation dose to the rectum during prostate cancer radiation. It separates the prostate and rectum and is naturally absorbed by the body in about six months.3 More than 50,000 patients worldwide have been successfully treated with SpaceOAR Hydrogel.7
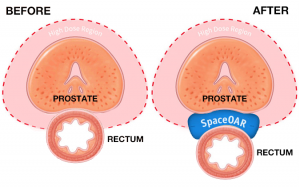
Why is SpaceOAR Hydrogel important for patients?
When treating prostate cancer patients with radiation therapy, the goal is to kill the cancer cells while avoiding damage to surrounding healthy tissue. The prostate is next to the rectum and naturally separated by a small space. Due to the proximity, prostate radiation therapy can unintentionally cause damage to the rectum, which can lead to issues with bowel function. SpaceOAR Hydrogel is a gel spacer that temporarily moves the rectal wall farther away from the prostate. By separating the prostate from the rectum, the radiation dose delivered to the rectum is reduced, which may lessen damage to the rectum.
Clinical data demonstrated the benefits of SpaceOAR Hydrogel, including a reduction of rectal injury resulting in maintained bowel function and a higher likelihood to maintain urinary and sexual function.10
What are the risks of SpaceOAR Hydrogel?
As with any medical treatment, there are some risks involved with the use of SpaceOAR Hydrogel. Potential complications include, but are not limited to: pain associated with SpaceOAR Hydrogel injection; pain or discomfort associated with SpaceOAR Hydrogel; needle penetration of the bladder, prostate, rectal wall, rectum or urethra; injection of SpaceOAR Hydrogel into the bladder, prostate, rectal wall, rectum or urethra; local inflammatory reactions; infection; injection of air, fluid or SpaceOAR Hydrogel intravascularly; urinary retention; rectal mucosal damage, ulcers, necrosis; bleeding; constipation; and rectal urgency.
What is the gel made of? Is it safe?
SpaceOAR Hydrogel is made up of two liquids that, when combined, form a soft gel material mostly made of water. Studies have shown that the material is biocompatible, can be used in the body without causing injury or a reaction and can be safely absorbed by the body. The material that the SpaceOAR Hydrogel is made from has been used in other implants such as surgical sealants used in the eye, brain and spine.
Where is the procedure done and how long does it take?
SpaceOAR Hydrogel can be placed in a short visit in our office or another outpatient setting.
In what type of radiation treatment can SpaceOAR Hydrogel be used?
SpaceOAR Hydrogel can be used in all types of radiation therapy for the prostate.
How soon after the procedure can patients return to their normal activities?
Patients are usually able to go back to normal activities shortly after SpaceOAR Hydrogel is implanted.
How long does the hydrogel remain in the body?
SpaceOAR Hydrogel stays in place for about three months. After about six months, the hydrogel is naturally absorbed into the body and removed through urine.
Is SpaceOAR Hydrogel covered by insurance?
SpaceOAR Hydrogel is covered by many insurance plans and is reimbursed by Medicare on a case-by-case basis. Patients should verify their benefits with their insurance company in advance of the scheduled procedure.
Additional Resources
Click here to view the SpaceOAR Digital Brochure
Click here to view Patient Support and Advocacy Groups
- Leading Cancer Cases and Deaths, Male, 2015. Centers for Disease Control and Prevention. Gis.cdc.gov/Cancer/USCS/DataViz.html. Accessed February 25, 2019.
- Treatment for Prostate Cancer: External-Beam Radiation Therapy. Prostate Cancer Foundation. https://www.pcf.org/c/treatment-for-prostate-cancer-external-beam-radiation-therapy/. Accessed February 13, 2019.
- Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: Dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015 Aug 1;92(5):971-7.
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017 Apr 1;97(5):976-85.
- Hamstra D, Shah D, Kurtzman S, et al. Evaluation of sexual function on a randomized trial of a prostate rectal spacer. J Clin Oncol. 2017 February 20;35(Suppl 6):69.
- Survival Rates for Prostate Cancer. American Cancer Society. https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survival-rates.html. Accessed February 13, 2019.
- Data on file with Boston Scientific.
- Potential Side Effects of a Radical Prostatectomy. SpaceOAR Hydrogel. https://www.spaceoar.com/patients/prostate-cancer-resources-and-articles/potential-side-effects-of-a-radical-prostatectomy/. Accessed July 12, 2019.
- Reviewing the Most Common Treatment Options. SpaceOAR Hydrogel. https://www.spaceoar.com/patients/prostate-cancer-resources-and-articles/reviewing-the-most-common-treatment-options/. Accessed July 12, 2019.
- Hamstra DA, Mariados N, Sylvester J, et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase 3 trial. Pract Radiat Oncol. 2018 Jan – Feb;8(1):e7-e15.
