West Cancer Center is the first center in the country to offer the first FDA-approved Mesothelioma treatment in more than 15 years
A clinical study showed that the NovoTTF-100L™ System in combination with chemotherapy may help people with malignant pleural mesothelioma extend their lives
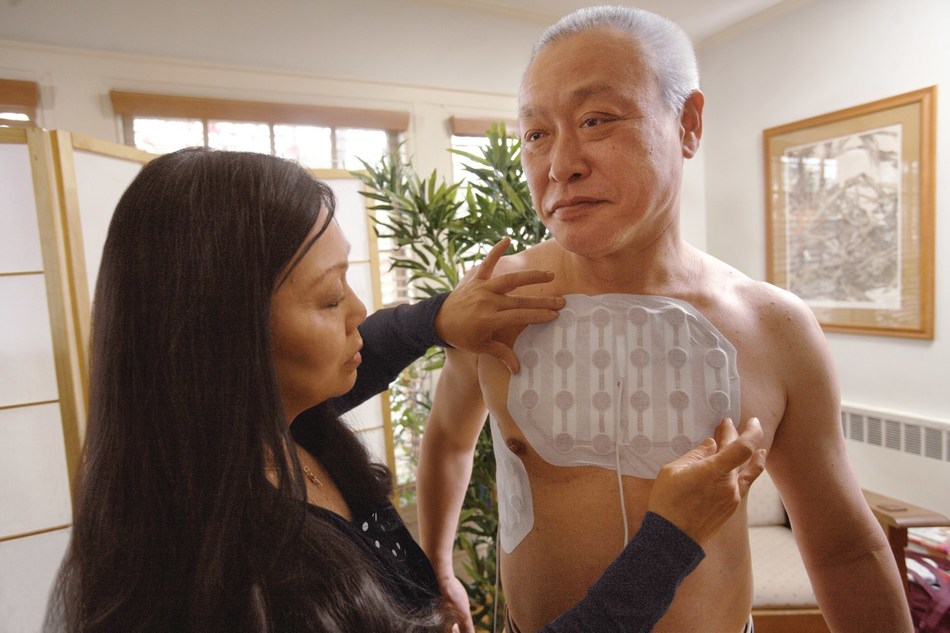
West Cancer Center is pleased to announce they have been designated as the first center in the country approved to prescribe NovoTTF-100L for the treatment of malignant pleural mesothelioma (MPM). Experts at West Cancer Center are now certified to prescribe this wearable and portable medical device, the first FDA-approved mesothelioma treatment in more than 15 years.
NovoTTF-100L is approved for the first-line treatment of adult patients with unresectable, locally advanced or metastatic MPM to be used concurrently with pemetrexed and platinum-based chemotherapy. In a clinical study, MPM patients treated with NovoTTF-100L plus chemotherapy experienced a median overall survival of 18.2 months.
“Many people with MPM are not candidates for surgery, and approved, non-surgical treatments are very limited,” said Matthew Ballo, MD, FACR, Director of Radiation Oncology at West Cancer Center. “We are really proud that West Cancer Center is the first to offer this therapy as part of a combination treatment, giving another, much-needed option to patients and their families. We actually have just prescribed NovoTTF-100L for one of our patients and he will be the first in the country to receive this treatment.”
NovoTTF-100 is a non-invasive, antimitotic cancer treatment that delivers Tumor Treating Fields (TTFields) to the region of the tumor. TTFields therapy uses electric fields tuned to specific frequencies to target and directly disrupt dividing tumor cells, inhibiting tumor growth and possibly causing affected cancer cells to die while sparing normal, healthy cells.
NovoTTF-100L for MPM is classified as a Humanitarian Use Device (HUD) and was approved under the Humanitarian Device Exemption (HDE) pathway. The HDE pathway was created to encourage companies to innovate in rare diseases with underserved patient populations. The FDA approved Optune®, another Tumor Treating Fields delivery system, under the Premarket Approval (PMA) pathway in 2011 for the treatment of recurrent glioblastoma (GMB) and in 2015 for the treatment of newly diagnosed GMB in combination with temozolimide. Since 2011, more than 10,000 patients with GMB have been treated with Tumor Treating Fields.
About Malignant Pleural Mesothelioma
Malignant pleural mesothelioma (MPM) is a rare cancer that has been strongly linked to asbestos exposure. Approximately 3,000 people are diagnosed with MPM in the United States annually. Prior to the FDA approval of NovoTTF-100L, pemetrexed plus cisplatin was the only FDA-approved therapy for patients with MPM that could not be surgically removed.